Inductively Coupled Plasma Mass Spectrometry (ICP-MS) stands as a cornerstone technique in elemental analysis, renowned for its sensitivity and versatility. From environmental monitoring to semiconductor manufacturing, ICP-MS provides crucial quantitative data across diverse fields. However, the journey to accurate and reliable ICP-MS results hinges significantly on sample preparation. Inadequate sample preparation can introduce a cascade of problems, including signal drift, elevated background noise, compromised detection limits, and unexpected interferences, ultimately undermining the integrity of your analysis. This article serves as a comprehensive guide to optimizing your sample preparation workflow, with a spotlight on leveraging assisted sample preparation tools to streamline processes and enhance data quality.
The primary interface of ICP-MS is liquid samples. Therefore, for most solid samples, dissolution into a suitable diluent is a prerequisite. While aqueous diluents like ultrapure water and dilute acids are preferred for their ease of handling, organic solvents become necessary for analyzing elemental impurities in matrices such as active pharmaceutical ingredients. When direct dissolution is not feasible, digestion methods, including hot block digestion, fusion techniques (e.g., lithium metaborate fusion), or microwave-assisted digestion, become indispensable. However, traditional digestion methods are often time-consuming, reagent-intensive, and carry the risk of contamination from reagents or insufficiently cleaned digestion vessels.
Irrespective of the chosen digestion method, the ideal outcome is a clear, particle-free solution ready for introduction into the ICP-MS instrument. Prior to analysis, several critical parameters demand careful consideration. The Total Dissolved Solids (TDS) content, calculated as the ratio of sample mass to the final solution volume, is a crucial factor. ICP-MS instruments have specific TDS tolerance limits, typically lower than ICP-OES. The residual acid concentration and the acid mixture itself can also exert a significant influence on instrument performance and the configuration of the sample introduction system. The presence of hydrofluoric acid (HF), for instance, necessitates the replacement of quartz components with inert alternatives. Ideally, the residual acid concentration in the digested sample should remain below 5% (v/v).
Direct Solid Sample Analysis: The Power of Laser Ablation
Direct solid sample analysis presents an attractive alternative in specific scenarios, encompassing conductive materials (metals, semiconductors), non-conductive samples (minerals, paper, plastics), and biological tissues. Laser Ablation (LA)-ICP-MS emerges as a powerful assisted sample preparation tool in this domain. By focusing a high-intensity pulsed laser onto the sample surface, LA directly converts solid material into an aerosol, which is then transported to the ICP-MS for ionization and analysis.
LA-ICP-MS offers several compelling advantages. It eliminates the need for hazardous digestion chemicals, minimizing contamination risks associated with sample handling and reagents. The small laser spot size (typically around 200 μm) makes LA a quasi-non-destructive technique, particularly valuable when analyzing precious or limited samples. Furthermore, LA-ICP-MS enables spatially resolved analysis, providing information about the distribution of analytes across the sample surface – a crucial capability for geological and biological sample characterization.
However, it’s important to acknowledge the limitations. Sample homogeneity becomes a critical factor when using LA-ICP-MS for bulk concentration measurements. Careful selection of the sampling area is essential to ensure representative data.
Consider Acid and Vial Purity!
ICP-OES and ICP-MS are inherently sensitive techniques designed to detect elemental impurities at trace and ultratrace levels. This sensitivity underscores the critical need to minimize background contributions from all sources. Elevated backgrounds can lead to false positive results and compromised detection limits, triggering unnecessary follow-up investigations in routine laboratories.
While some elements, like rare earth elements, have naturally low environmental abundance, minimizing background contamination, other elements, particularly alkali, alkaline earth, and transition metals (e.g., sodium, potassium, iron, copper, zinc), are prone to leaching from plasticware, including vials and vial caps. When switching vial batches or brands, conducting a preliminary leach test to assess vial purity is highly advisable.
Acid purity is another critical consideration. Acids and reagents commonly used in elemental analysis, such as nitric acid, hydrochloric acid, and hydrogen peroxide, are available in various purity grades. For ultratrace analysis using ICP-MS, only the highest purity acids should be employed.
Sub-boiling distillation offers a cost-effective approach to purify lower-purity acids, enhancing their suitability for trace analysis. Similarly, the water used for diluent preparation can also contribute to background contamination. Regular checks for trace elements in the water supply, along with routine maintenance of the water purification system, are essential. For trace element analysis, water with a resistivity of 18.2 MΩ cm is recommended whenever feasible.
Choose Your Acids Wisely!
Microwave-assisted digestion is frequently employed as an assisted sample preparation tool to facilitate the dissolution of challenging sample matrices. Microwave digestion enables digestions at elevated temperatures and pressures, accelerating decomposition. However, selecting the appropriate acid or acid mixture remains crucial for effective digestion and stabilization of target analytes.
Nitric acid (HNO3), while commonly used as a diluent, is often insufficient for sample digestion alone. Combining nitric acid with hydrogen peroxide (H2O2) effectively digests many organic matrices, such as food and feed samples. Perchloric acid can further enhance the oxidation potential of the mixture, but requires extreme caution due to its potential for violent reactions with organic materials. Hydrochloric acid (HCl) is beneficial for dissolving and stabilizing certain inorganic materials and critical analytes like mercury and platinum group metals. Aqua regia (a 3:1 mixture of hydrochloric acid and nitric acid) is particularly effective for dissolving metallic samples. Ensuring a sufficiently high HCl concentration (≥ 2%) is crucial for stabilizing mercury by converting initially formed precipitates (HgCl or HgCl2) into soluble chloro complexes ([(HgCl4)]2-).
Figure 1 (below) summarizes commonly used acids in digestion methods. Sulfuric acid can be useful for specific sample types digested on a hotplate or in high-pressure digestion systems, but its use with Teflon vessels in microwave digestion is contraindicated due to Teflon’s lower melting point than sulfuric acid’s boiling point at atmospheric pressure. Furthermore, sulfuric acid can introduce sulfur-based interferences in ICP-MS, limiting its general applicability.
Figure 1: A chart outlining various acids commonly utilized in sample digestion processes for ICP-MS analysis, including Nitric Acid (HNO3), Hydrochloric Acid (HCl), Hydrogen Peroxide (H2O2), Perchloric Acid (HClO4), Aqua Regia (HNO3:HCl), and Sulfuric Acid (H2SO4), detailing their applications and considerations.
Always prioritize personal protective equipment when handling acids and hazardous chemicals. Consult microwave digestion system manufacturers for detailed guidance on acid selection, sample and acid amounts, and optimized temperature programs.
Dilute Your Sample Smartly!
High TDS content in samples can induce signal suppression and drift in ICP-MS. The typical TDS limit for ICP-MS is between 0.2 and 0.5% (m/v). Consequently, many samples suitable for direct ICP-OES analysis require dilution prior to ICP-MS analysis. Specialized sample introduction accessories, such as argon gas dilution systems, can enable the aspiration of solutions with TDS concentrations exceeding 3-4%. However, this on-line dilution inherently reduces detection sensitivity. The primary advantage of these accessories lies in eliminating manual dilution steps, saving operator time, reducing diluent consumption and waste, minimizing contamination risks, and mitigating human error. However, because the dilution gas influences plasma conditions, the dilution factor must remain constant across all standards and samples within a batch. Plasma stabilization times become critical after any changes in plasma conditions to prevent instrumental drift. This inflexibility can be a drawback when analyzing diverse sample types. Setting a high dilution factor to accommodate high-matrix samples unnecessarily dilutes low-matrix samples, leading to poorer method detection limits (MDLs) and compromised accuracy for those samples.
Automated liquid dilution systems, integrated with valve systems and autosamplers, offer a flexible solution. Controlled by ICP-MS software, these assisted sample preparation tools enable pre-defining fixed or variable dilution factors (up to several hundred-fold) for each sample. Intelligent autodilution capabilities allow the system to automatically dilute and re-analyze samples exhibiting internal standard suppression exceeding user-defined limits or concentrations falling outside the calibration range. Re-analysis data, along with the applied dilution factor, are meticulously documented for regulatory compliance. Intelligent autodilution also streamlines calibration curve generation. A multi-point calibration curve spanning several orders of magnitude can be automatically generated from a single high-concentration stock solution using online autodilution.
Manage Your Backgrounds!
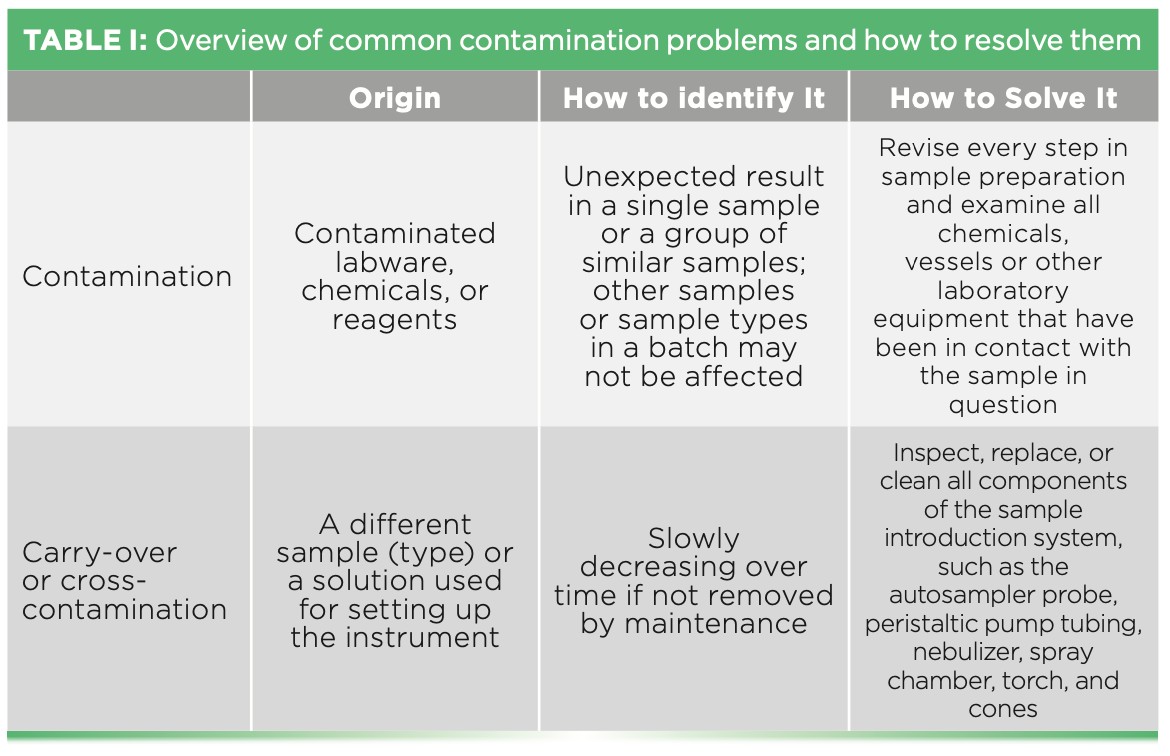
Background contamination from laboratory equipment, chemicals, or carry-over between samples represents a significant concern. Equipment and chemical contamination necessitate rigorous testing of all consumables and reagents. Carry-over contamination requires implementing effective cleaning strategies between analyses. For digested samples, a blank digestion, mirroring the sample preparation procedure but omitting the sample, should be included with each batch. This blank digestion helps identify and rectify contamination originating from the digestion process before biased results are generated. Table I (below) summarizes common sources of contamination and carry-over in ICP-MS analysis.
Table 1: A table outlining common causes of biased analytical results in ICP-MS due to contamination, categorized into sources like reagents, labware, environment, and carry-over, along with preventative measures for each source.
Dilute and Shoot!
Frequently, limited prior knowledge about a sample matrix can present analytical challenges. Unexpected matrix elements or interferents may only become apparent during sample analysis, leading to perplexing results and potential contamination of subsequent samples. To proactively address such challenges, a “dilute and shoot” approach, coupled with a full mass scan, is recommended for unknown samples.
Running a full mass scan provides a comprehensive overview of the sample’s elemental composition, revealing highly concentrated matrix elements or unexpected interferences. A slightly higher initial dilution than anticipated offers a safety margin against persistent contamination. Unexpected interferences can arise from various sources. Doubly charged ions, formed when an element’s second ionization potential is lower than argon’s ionization potential, can appear at half mass in the spectrum. Rare earth elements, particularly neodymium (Nd), samarium (Sm), and gadolinium (Gd), can generate false positive signals for key contaminants like arsenic (150Nd++, 150Sm++) and selenium (156,160Gd++ on 78,80Se+). While mathematical corrections can mitigate doubly charged interferences in specific concentration ranges, triple quadrupole ICP-MS systems offer superior interference removal capabilities. Doubly charged interferences can also impact internal standards. For example, high lead concentrations can cause 206Pb++ interference on 103Rh+, a common internal standard, potentially affecting the accuracy of all analytes standardized against 103Rh. Kinetic Energy Discrimination (KED), while effective for suppressing polyatomic interferences in the lower and middle mass range, becomes less effective at higher masses due to diminishing relative mass differences between analytes and polyatomic interferences. Even low concentrations of interfering elements can introduce bias. For instance, tungsten oxide interference can affect mercury analysis even at single-digit μg/L tungsten concentrations.
Summary
A critical evaluation of every step in the sample preparation protocol is paramount for achieving successful, accurate, and reliable elemental analysis using ICP-MS. Thoroughly assessing consumables and reagents, implementing appropriate blank digestions, and incorporating quality control (QC) checks are essential for identifying and resolving potential sources of biased results. Embracing assisted sample preparation tools like microwave digestion and automated dilution systems further streamlines workflows, enhances data quality, and ensures the robustness of your ICP-MS analyses.
Daniel Kutscher, Jianfeng Cui, and Cristian Cojocariu are with Thermo Fisher Scientific in Bremen, Germany. Direct correspondence to: [email protected] ●